People with obesity-related heart failure and diabetes can get substantial heart-health benefits from weight-loss drug Wegovy, even if they don’t shed many pounds on the medication, according to new research.
Source link
loss
Bitcoin Supply In Loss Hits 10% After Crash: What Happened Last Time
On-chain data shows the Bitcoin supply in profit has plunged following the latest crash in the asset’s price towards the $65,000 level.
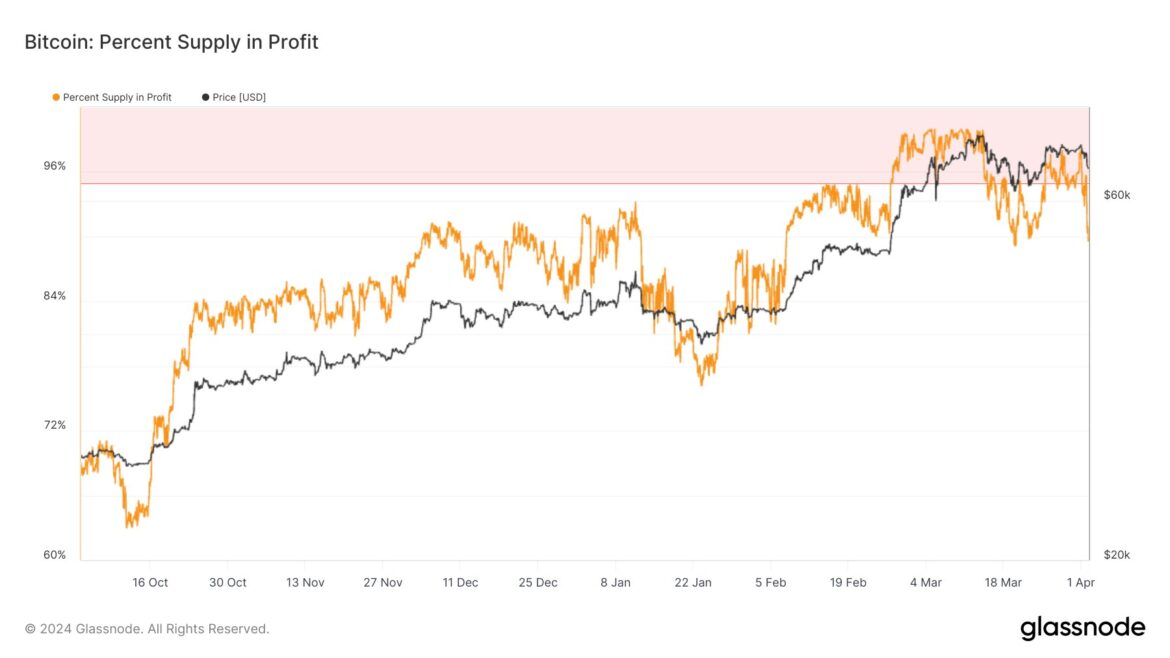
Bitcoin Supply In Profit Is Now Down To Around 90%
As analyst James Van Straten pointed out in a post on X, around 10% of the BTC supply is now in a state of loss. The on-chain indicator of interest here is the “Percent Supply in Profit,” which tracks the percentage of the total circulating Bitcoin supply holding an unrealized gain.
This metric works by going through the blockchain history of each coin in circulation to see the price at which it was last transferred. Assuming that this previous transaction involved a change of hands, the price at its moment would serve as the cost basis for the coin.
The coins with a cost basis that is less than the current spot price of the cryptocurrency would naturally be considered to be holding a profit, and as such, they would be counted under the supply in profit.
The Percent Supply in Profit adds up all such coins and calculates what part of the total supply they make up for. The opposite metric, the Percent Supply in Loss, adds up the coins not satisfying this condition.
Since the total circulating supply must add up to 100%, the Percent Supply in Loss can be deduced from the Percent Supply in Profit by subtracting its value from 100.
Now, here is a chart that shows the trend in the Percent Supply in Profit for Bitcoin over the last few months:

Looks like the value of the metric has taken a plunge in recent days | Source: @jvs_btc on X
As displayed in the above graph, the Bitcoin Percent Supply in Profit has seen a sharp drop recently as the cryptocurrency price has gone through a significant drawdown.
The indicator’s value has dropped to around the 90% mark, which means that about 10% of the supply is currently carrying a loss. The chart shows that the last time the metric touched these levels was back on 22 March. Interestingly, the asset also found its bottom around then.
Earlier, the Percent Supply In Profit had pushed towards the 100% mark, which was a natural consequence of the price setting a new all-time high (ATH), since at fresh highs, all of the supply must be out of the red.
Generally, the investors in profit are more likely to sell their coins, so if many come into gains, the possibility of a mass selloff rises. Due to this reason, high levels of the Percent Supply In Profit have often led to tops.
Similarly, bottoms become more likely when investor profitability levels drop relatively low. The current value of 90% is still quite high, but this isn’t unusual during bull runs, as there is strong demand and ATHs are being explored.
The fact that the profitability has cooled off compared to earlier levels may be constructive for the rally’s chances to see a continuation, just like it did last month.
BTC Price
At the time of writing, Bitcoin has been trading at around the $65,700 level, down more than 5% over the past week.
The price of the asset seems to have been tumbling down over the past couple of days | Source: BTCUSD on TradingView
Featured image from Shutterstock.com, Glassnode.com, chart from TradingView.com
Disclaimer: The article is provided for educational purposes only. It does not represent the opinions of NewsBTC on whether to buy, sell or hold any investments and naturally investing carries risks. You are advised to conduct your own research before making any investment decisions. Use information provided on this website entirely at your own risk.

There may be a stronger case to invest in single stocks over exchange-traded funds in the weight loss space.
Amplify ETFs and Roundhill Investments each filed a prospectus last week to launch funds focused on weight loss companies, a move that Strategas ETF and technical strategist Todd Sohn believes hinges on the performance of two dominant stocks: Novo Nordisk (NVO) and Eli Lilly (LLY).
“The main holdings are going to be Lilly and Novo Nordisk, and probably one or two other big names … along with some of the manufacturers down the supply chain,” he told CNBC’s “ETF Edge” this week. “Ultimately, it’s up to those big behemoths that are playing those drugs.”
With just two players currently at the forefront of the U.S. obesity drug market, ProShares’ Simeon Hyman questions the relevance of weight loss ETFs for investors looking to buy into the industry.
“I think that’s one of the challenges whenever you see an innovation like this,” the firm’s global investment strategist said in the same interview. “If the benefits are going to incumbents, then maybe there isn’t a theme per se that needs to be exploited.”
Strategas’ Sohn also suggested that ETFs based on themes, rather than sectors or indices, might be falling out of favor with investors.
“I think thematics are a little bit on the backburner right now, especially the way they performed the last couple of years. I think there’s room for them, but more than one, it’s gonna be tough,” he said.
So far in 2024, Novo Nordisk has gained 29% and Eli Lilly is up 30%, as of Wednesday’s close. The broader Health Care Select Sector SPDR (XLV) is 7% higher during the same period.
Disclaimer

Shares of iRobot Corp. initially jumped 10% then cooled in after-hours trading Wednesday after the company posted quarterly results that topped analysts’ revenue and earnings estimates.
IRobot
IRBT,
reported a fiscal fourth-quarter net loss of $63.6 million, or $2.28 a share, compared with a net loss of $84.1 million, or $3.07 a share, in the same quarter a year ago. Adjusted earnings were a loss of $1.82 a share.
Revenue dropped to $307.5 million from $357.9 million in the year-ago quarter.
Analysts surveyed by FactSet had expected on average a net loss of $2.11 a share on revenue of $308 million.
The Roomba maker anticipates full-year 2024 revenue of between $825 million and $865 million, while FactSet analysts are forecasting $865 million.
After spiking in after-hours trading immediately following its earnings, shares lost their gains and ended the extended session flat. Shares of iRobot have plunged 71% over the past year, while the broader S&P 500 index
SPX
is up 27%.
Last month, Amazon.com Inc.
AMZN,
called off its planned acquisition of iRobot following opposition from the European Union. IRobot also said it would lay off 31% of its employees, or about 350 people, and that its chief executive, Colin Angle, had stepped down.
In a recent investigation by The Guardian, alarming details have emerged regarding a crypto project, HyperVerse, that allegedly lost $1.3 billion of investors’ funds.
The report reveals that the chief executive officer promoted by the project, supposedly backed by celebrity endorsements including Chuck Norris, appears to be absent.
Investigation Exposes HyperVerse Crypto Scam
HyperVerse, promoted by Australian entrepreneur Sam Lee and his business partner Ryan Xu, founders of the now-collapsed Australian Bitcoin (BTC) company Blockchain Global, has been scrutinized for its deceptive practices. The project attracted thousands of investors, who ultimately lost millions of dollars.
The investigation raises concerns about the legitimacy of HyperVerse’s CEO, as the qualifications and credentials attributed to the supposed chief executive, Steven Reece Lewis, have no basis.
Promotional material released for HyperVerse claimed that Lewis graduated from the University of Leeds and held a master’s degree from the University of Cambridge. However, neither institution has any record of his existence.
Furthermore, there are no records of Lewis on the UK companies register, Companies House, or the US Securities and Exchange Commission (SEC). Interestingly, Adobe, a publicly listed company, also has no record of any acquisition involving a company owned by “Steven Reece Lewis.”
The report indicates that HyperVerse managed to secure celebrity endorsements, including video messages of support from Steve Wozniak, co-founder of Apple, and actor Chuck Norris.
However, it is unclear how these messages were obtained, as all four celebrities mentioned in the report are available for hire through Cameo, where individuals can pay to have high-profile individuals read scripted messages.
Australian Authorities Under Fire
The investigation also highlights regulatory concerns, as HyperVerse operated without significant scrutiny in Australia despite being flagged by regulators overseas as a possible scam or suspected pyramid scheme.
The Australian Securities and Investments Commission (ASIC) has been referred to the case but has not yet taken action.
Investors in HyperVerse were lured with promises of substantial returns and the opportunity to explore a new digital metaverse similar to Facebook. However, the scheme ultimately resulted in significant losses for investors, estimated at $1.3 billion in 2022, according to blockchain analysts Chainalysis.
The Guardian’s findings shed light on the deceptive practices employed by HyperVerse and raise questions about the responsibilities of regulators in overseeing such projects.
As the aftermath of this cryptocurrency scandal unfolds, investors and authorities alike are left grappling with the consequences of a scheme that capitalized on false claims and celebrity endorsements to defraud unsuspecting individuals.
Featured image from Shutterstock, chart from TradingView.com
Disclaimer: The article is provided for educational purposes only. It does not represent the opinions of NewsBTC on whether to buy, sell or hold any investments and naturally investing carries risks. You are advised to conduct your own research before making any investment decisions. Use information provided on this website entirely at your own risk.
Eli Lilly (LLY 0.12%) and Novo Nordisk (NVO -0.46%) have been the two best-performing large-cap pharma stocks over the past 10 years. The core reason is their strong competitive positions in type 2 diabetes and obesity care.
Lilly’s leading metabolic disease therapy is tirzepatide, which is sold under the brand names Mounjaro for type 2 diabetes and Zepbound for obesity care. Bullish analysts expect this lead metabolic therapy, combined with Lilly’s follow-on offerings in the space, to hit $65 billion in annual sales by 2031.

Image source: Getty Images.
Novo’s main product in this segment is semaglutide, which is available as Wegovy for weight loss, Ozempic as a once-weekly injection for type 2 diabetes, and Rybelsus as a once-daily tablet for type 2 diabetes.
Although Novo holds a first-mover advantage for this class of drugs in both the weight loss and type 2 diabetes settings, the Danish drugmaker is expected to essentially split these markets with Lilly by 2031.
Which of these supercharged growth stocks is the better investment option right now? Let’s examine them more closely to find out.
A traditional and nontraditional valuation comparison
Even though Novo and Lilly both operate in the pharmaceutical industry, these two healthcare titans have dramatically different approaches to value creation. Novo focuses primarily on diabetes and obesity, while Lilly has a more diversified portfolio of drugs. How do they compare in terms of valuation and innovation?
One way to compare them is to use a three-factor model, which estimates the future earnings of a company based on its growth rate, costs, and shareholder rewards. Using this method, Novo’s stock seems to be cheaper than Lilly’s, as it trades at a lower range of multiples for its projected 2031 earnings (midpoint estimate of 16.5) compared to those for Lilly’s stock (midpoint estimate of 28). However, this is a rough approximation that depends on several assumptions that may fail to pan out.
Another way to compare them is to look at their research and development (R&D) spending, which partially reflects their investment in innovation. Innovation is crucial for pharma companies, as their products have limited patent protection. What’s more, R&D spending can be seen as a simple, yet useful, proxy for the net present value (NPV) of a company’s pipeline, which is the sum of the projected cash flows from its clinical portfolio on a risk-adjusted basis.
Lilly has been investing heavily in R&D, spending about a quarter of its annual sales on developing new drugs over the past five years. Novo, on the other hand, has been more conservative, spending only about 13% of its sales on R&D over the same period.
This difference reflects the broader scope of Lilly’s clinical efforts relative to Novo’s over this period. Apart from diabetes and weight loss, Lilly has also been investing in multiple high-value areas such as cardiovascular care, immunology, neuroscience, and oncology. Novo, on the other hand, has been mainly focused on building on its dominance in type 2 diabetes treatment and gradually branching out into therapies for rare diseases.
Verdict
Lilly’s higher premium is arguably well deserved. The company’s substantially larger R&D spend as a fraction of total revenue, and resulting broader pipeline, make it less dependent on the future — and largely unknown — competitive dynamics of the type 2 diabetes and/or weight loss markets. So while Novo’s more specialized approach to pipeline development should translate into a solid competitive position in these high-profile disease settings, Lilly’s stock is arguably the better buy because of the company’s broader and less risky approach to growth.
Sopa Images | Lightrocket | Getty Images
Pfizer’s twice-daily version of its experimental weight loss pill has now joined a long list of other scrapped drugs that aimed to treat obesity but came with unintended consequences.
The drugmaker on Friday said it will stop developing the twice-daily treatment, danuglipron, after obese patients taking the drug lost significant weight but experienced high rates of adverse side effects in a midstage clinical trial. Pfizer noted that it will release data on a once-daily version of the pill next year, which will “inform the path forward.”
The announcement came six months after Pfizer scrapped a different once-daily pill in June, citing elevated liver enzymes. Pfizer’s move to drop two obesity drug candidates in just a few months demonstrates how difficult it is to develop an effective, safe and tolerable treatment for losing weight, even after recent breakthrough medications entered the space.
That includes Novo Nordisk‘s Wegovy and diabetes treatment Ozempic as well as Eli Lilly‘s diabetes drug Mounjaro. They have all skyrocketed in popularity — and slipped into shortages — over the last year for safely and successfully causing significant weight loss. An estimated 40% of U.S. adults are obese, making those drugs the pharmaceutical industry’s newest cash cow.
But before the current weight loss industry gold rush, the path to treating obesity was strewn with failures dating back decades.
The main reason many experimental treatments were scrapped by drugmakers, rejected by U.S. regulators or eventually pulled from the market were unintended side effects, including elevated liver enzymes, cancer risks, cardiovascular risks and serious psychiatric problems, such as suicide.
Eisai’s lorcaserin
One of the most recent casualties among experimental obesity drugs is Japanese drugmaker Eisai’s lorcaserin, which was removed from the market in 2020 due to causing an increased risk of cancer in patients.
The Food and Drug Administration greenlit lorcaserin in 2012 based on several clinical trials but required Eisai to conduct a larger and longer study on the drug after the approval.
That study on about 12,000 patients over five years found that more people taking lorcaserin were diagnosed with cancer compared with those taking a placebo, which led the FDA to pull the drug from the market.
Lorcaserin, marketed under the brand name Belviq, didn’t appear to gain much traction while it was commercially available. In its full-year 2019 earnings, Eisai reported that Lorcaserin had sales of $28.1 million in the U.S. for the year. Global sales of the drug were about $42 million. Eisai’s total sales for the year were roughly $4.42 billion.
Sanofi’s rimonabant
An obesity drug called rimonabant from Sanofi and Aventis was withdrawn from all markets in 2008 due to the risk of serious psychiatric problems, including suicide.
Notably, the treatment never won approval in the U.S. because a panel of experts to the FDA rejected the drug amid fears that it may cause suicidal thoughts. But European regulators approved rimonabant, marketed under the name Acomplia, in 2006 based on extensive clinical trials.
Two years later, European regulators recommended the suspension of rimonabant after one of its committees determined that the risks of the treatment — particularly psychiatric issues — outweighed its benefits.
The treatment suppressed appetite by blocking the receptor of cannabinoid substances in the brain, which plays an important role in regulating the body’s food intake and metabolism.
Due to rimonabant’s limited time on the market and failure to win U.S. approval, the drug never reached Sanofi’s lofty projection that it would eventually generate $3 billion a year or more.
Abbott Laboratories’ sibutramine
Several obesity drugs have also been discontinued, rejected or pulled from the market due to unintended cardiovascular risks.
That includes sibutramine from Abbott Laboratories, which was once widely used as a treatment for obesity along with diet and exercise.
The drug was first approved in 1997, but carried warnings about high blood pressure and a risk of heart attack and stroke in cardiovascular patients.
A large, long-term trial on nearly 10,000 adults confirmed that sibutramine was associated with a significant increase in cardiovascular events, which prompted regulators in the U.S. and Europe to pull the drug from those markets in 2010.
Sales of sibutramine had been dwindling ahead of its removal from the market. The drug raked in only $80 million globally, including $20 million from the U.S., in the first nine months of 2010.
Recent evidence suggests that the newest slate of approved weight loss drugs may have the opposite effect on heart health: Weekly injections of Wegovy slashed the overall risk of heart attack, stroke and death from cardiovascular causes by 20%, according to a recent clinical trial.
Dow on track for third straight monthly loss as investors await Wednesday Fed decision
U.S. stocks edged higher early afternoon on Tuesday, as investors prepare for major events later in the week including the latest Federal Reserve interest rate decision and new jobs numbers.
How stocks are trading
-
The Dow Jones Industrial Average
DJIA
rose 28.7 points, or 0.1%, to 32,951 -
The S&P 500
SPX
gained 8 points, or 0.2%, to 4,175 -
The Nasdaq Composite
COMP
added 5 points, or less than 0.1%, to 12,796
All three major indexes were on track for October declines, which would mark a third straight month of declines.
What’s driving markets
Traders are attempting to extend stock markets’ Monday rally, and they may be looking past a difficult October with big events on the horizon.
The third-quarter earnings-reporting season rumbles on — and it’s been a rocky start for some companies Tuesday morning.
Caterpillar Inc.
CAT,
shares dropped 6%, leading Dow decliner and dragging on the blue-chip gauge. Even with a third-quarter profit beat, the maker of construction mining equipment has a tepid outlook for the fourth quarter.
The company’s performance is a cause for broader caution, according to Steve Sosnick, chief strategist at Interactive Brokers. It’s just one company, he noted — but it may be reflecting “a pretty negative story about cyclicals right now.”
Pfizer Inc.
PFE,
shares were also lower after a wider-than-expected loss, although the pharmaceutical maker did reaffirm its full-year outlook.
The most widely anticipated earnings event this week is Thursday after the bell, when Apple Inc.
AAPL,
reports its numbers.
Then there’s the focus on the Treasury market. The 10-year Treasury yield
BX:TMUBMUSD10Y
dropped 2.3 basis points to 4.87% Tuesday afternoon.
News on Monday that the U.S. Treasury was planning to borrow less than expected this quarter and would thus have to issue less paper was one factor for bond prices. The Treasury will announce its third-quarter refunding program on Wednesday.
Another factor helping suppress Treasury yields, and therefore possibly helping sentiment in equities, was data showing that manufacturing in China unexpectedly slipped back into contraction in October.
Such signs of a struggling global economy will be part of the Federal Reserve’s calculations as it begins its two-day policy meeting on Tuesday. It is expected to leave its policy interest rates unchanged at a range of 5.25% to 5.5%.
Central bankers and investors have more information Tuesday on labor costs for employers. Worker compensation increased 1.1% during the third quarter, higher than the expected 1%. Labor costs have increased at least 1% for nine straight quarters.
In a hard look at the numbers, Cory Stahle, an economist at Indeed Hiring Lab, said wage growth is slowing.
“When workers with more volatile compensation are removed (those paid on commission, for example) the slowing trend is more clear,” he said. “Today’s data is a mixed bag for Federal Reserve policy makers — things are headed in the right direction, but maybe not at the pace they are hoping for.”
The employment cost report “definitely is not market-friendly, especially ahead of a Fed meeting” where the central bank is focused on the interplay between labor costs and inflation, said Interactive Brokers’s Sosnick.
Also Tuesday, new data showed home prices continuing to rise for the sixth straight month in the country’s 20 biggest metropolitan housing markets, even as mortgage rates inch closer to 8%. The S&P CoreLogic Case-Shiller 20-city house-price index increased 1% in August from July. On an annualized basis, prices in the major markets climbed 2.2%.
New numbers on consumer confidence out Tuesday reached a five-month low, buffeted by the headwinds of rising interest rates, inflation worries and uncertainty about the Israel-Hamas war. The 102.6 read in October slipped from a revised 104.3 in September, according to the Conference Board. Economists polled by The Wall Street Journal were expecting a read of 100.
Companies in focus
-
JetBlue Airways Corp.’s stock
JBLU,
-10.97%
fell 12.9% after the carrier warned it would post a wider-than-expected fourth-quarter loss, while it missed analyst estimates for its third-quarter loss and revenue. -
BP shares
BP,
-4.96% BP,
-4.58%
dropped 4.5% on Tuesday after the British oil major fell short of analysts’ expectations in posting a 60% drop in its third-quarter profits, following a weak performance from its gas-trading division.
— Jamie Chisholm contributed.
Weight loss has always been big business, but it’s exploded of late due to surging demand for Ozempic, Wegovy and other new diabetes and obesity drugs.
In the first half of 2023, sales of Ozempic and Wegovy rose by 58% and 363%, respectively. That’s after quarterly prescriptions for those types of GLP-1 treatments, which mimic a hormone in the gut to suppress a person’s appetite, increased 300% between early 2020 and the end of last year.
But as consumers and businesses pour more money and resources into tackling the obesity epidemic, which costs the U.S. more than $170 billion a year, drug developers aren’t alone in coming up with innovative solutions.
Signos, a five-year-old startup, is taking an approach that doesn’t involve pills.
The company is using off-the-shelf continuous glucose monitors, or CGMs, and providing real-time diet and exercise recommendations based on an individual’s readings. CGMs are small sensors worn on the upper arm that track glucose levels, primarily for people with diabetes. The information is wirelessly sent to a smartphone, allowing the user to better prevent emergencies.
Signos uses CGMs built by Dexcom. The startup has its own app that shows users how their body responds to specific foods, what causes their glucose to spike and when they should exercise to get the best results for weight loss.
On Tuesday, Signos said it closed a $20 million funding round led by Cheyenne Ventures and GV, formerly known as Google Ventures. Dexcom Ventures also contributed to the financing. Signos said it will use the fresh capital to continue its research into metabolic health and to expand its team, which is currently around 45 people.
“Whether you have five pounds to lose or 100, we want to make sure we’re able to help everybody,” Sharam Fouladgar-Mercer, Signos’ co-founder and CEO, told CNBC in an interview.
Customers who sign up for Signos can choose a one-month, three-month or six-month plan. With the half-year plan, users pay $143 a month, which includes all of the pricey CGMs they’ll need during that time. The company declined to share specific details about how many people are currently using its platform.
Fouladgar-Mercer said the long timelines are designed to attract users who are serious about their weight-loss journey. Additionally, the sensors themselves have a long wear time. The Dexcom G6 and G7, the latest devices, can measure glucose for up to 10 days. Signos currently supports the G6 and will soon work with the G7 as well.
Fouladgar-Mercer said Signos is using Dexcom’s CGMs as part of a clinical study approved by an institutional review board designated by the U.S. Food and Drug Administration to monitor biomedical research involving real people.
Fouladgar-Mercer said he created the company in 2018 partly because of his own struggle to manage weight throughout his life. He trained as an athlete and played hockey in college, but he said he noticed how food often affected him differently from the way it affected his teammates.
He said he always felt that, in an effort to understand an individual’s metabolism, there was a “critical component” missing, and it had been nagging at him for 30 years.
Signos helps users understand the right decision to make in the moment, but they can go “behind the scenes” and learn as much about the science as they’d like, Fouladgar-Mercer said. Users can also integrate sleep data, heart rate data, and exercise data from their Apple Watch to personalize their profile even more.
“Once they trust the system works and they understand the methodology, they can just follow the really quick, here’s what I do, here’s what I do, here’s what I do,” Fouladgar-Mercer said. “And that’s how you get behavioral change.”
Though Dexcom primarily develops its CGMs for patients with diabetes, the company is also working toward broader applications. For instance, next year it’s releasing a new product meant for people who aren’t taking insulin. Similarly, Abbott Laboratories, which dominates the global CGM market, is hoping to bring its first consumer-facing CGM, called Lingo, to the U.S. next year, adding personalized coaching with recommendations about diet, sleep and exercise.
Fouladgar-Mercer said Signos has more data points than “anybody does in the world for non-diabetics.” He added that since the company built its first product almost five years ago, it’s been able to focus on fine-tuning its technology.
“I don’t want to incorrectly set expectations,” Fouladgar-Mercer said. “I think a lot of times, it’s like, ‘Oh, lost X pounds in X days.’ That’s not what we’re trying to accomplish. It’s really, how do we put you on a sustainable journey? And that journey is not going to be done in two or three days.”
Fouladgar-Mercer said Signos can work well alongside Ozempic and Wegovy from Novo Nordisk and other GLP-1 treatments. Novo Nordisk’s share price has quadrupled since 2018, and the company is now the most valuable in Europe.
Fouladgar-Mercer said GLP-1 drugs are a “powerful tool” that can help people jump-start weight loss, but it can be challenging to keep weight off if they stop taking the medication. Platforms such as Signos can help to reinforce and maintain a healthier lifestyle over time, he said.
Ultimately, he said, he wants people to use Signos to learn how to make better choices that work best for their bodies.
Signos, Fouladgar-Mercer said, can use technology and data “to drive behavioral change, and then wrap that all in a system that really is focused on driving and solving this biggest problem we have in America, which is weight.”
WATCH: Novo Nordisk stops Ozempic trial early after signs of success